Machavert Pharmaceuticals RAL GTPase Inhibitors Suppress KRAS Cancer Metastasis in Preclinical Animal Model
– Machavert hits its preclinical proof of principle milestone that small molecule RAL GTPase inhibitors suppress KRAS mutant cancer metastasis in vivo –
Aurora, Colo, September 22, 2020 – Machavert Pharmaceuticals, a preclinical stage pharmaceutical company focused on precision medicine cancer therapies, has established that RAL GTPase (RAL) inhibitors suppress metastasis formation and spread in an KRAS G12C pancreatic cancer animal model. This represents an important proof of principle milestone for Machavert to achieve in vivo confirmation that RAL inhibitors can suppress KRAS mutant cancer metastasis.
The preclinical results on metastasis formation were obtained in an orthotopic pancreatic cancer model where a tumor xenograft is surgically implanted directly onto the pancreas of test mice to form a clinically relevant model that mimics clinical metastasis formation in human patients. A summary of experimental results follow:
• Human MIA PaCa-2 pancreatic cancer KRAS G12C tumors where implanted onto the mice pancreas and then evaluated for inhibition of the presence of metastases by the RAL GTPase inhibitor, MP2018. At the end of the experiment, 7 out of 9 animals did not form metastasis in a group of mice treated with MP2018. In a control group not treated by MP2018 there were metastases in 6 out of 9 animals. On average, the MP2018 treated group had more than five times less metastases formed per single mouse than in the control group.

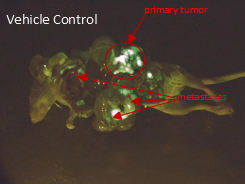
The figure above depicts mice with tumors and metastases labeled by green fluorescent protein (GFP) dye. The panel on the left shows one mouse from the MP2018 group, and the panel on the right shows a mouse from a control group with multiple metastases.
• Additionally, in a companion experiment, MP2018 inhibited growth of primary KRAS G12C tumors with the MIA PaCa-2 KRAS G12C xenograft model grown on the flanks of mice. MP2018 treatment achieved both a tumor growth delay efficacy and increased mouse survival efficacy with a high degree of statistical significance (p<0.0001).
Machavert will further advance the new class of cancer therapeutics, RAL GTPase inhibitors, targeting KRAS mutant cancers. The RAL inhibitors were discovered at the University of Colorado School of Medicine (CU), and relevant research was published in Nature. Machavert has been developing the RAL inhibitors since it entered into an exclusive option and evaluation license agreement in 2018 and later an exclusive worldwide license in 2019 with the University of Colorado for its RAL related intellectual property.
KRAS mutant cancers are linked to nearly one-third of all cancers and are challenging to treat. The novel RAL inhibition approach addresses treatment complexities and provides promise for anti-cancer therapy. RAL GTPases are effectors of RAS signaling and play a role in tumor proliferation, survival, and metastasis. A significant advantage of targeting RAL is the applicability against multiple subtypes of KRAS mutations and the potential to inhibit tumor metastasis and recurrence.
“Drugging the RAL GTPase pathway offers promise to achieving efficacy against multiple human cancers, including pancreatic, colon, and lung cancer. This unique therapeutic approach will be advantageous for patients who currently have few treatment options for KRAS mutant cancers,” said Dr. Neal Goodwin, Chief Scientific Officer of Machavert. „The therapeutic utility of these molecules can be additive to other frontline therapies, including other targeted KRAS therapeutics in development to prevent metastasis across multiple solid tumor therapeutic areas.” This synergy is exciting since recent developments within with KRAS G12C inhibitors in development entail that successful treatment of KRAS mutant cancers is being pursued on multiple fronts with combination therapies. “I see RAL inhibitors as complementary to the other therapeutic approaches against KRAS cancers by functioning to treat and inhibit metastasis,” added Dr. Goodwin.
MP2018 is further being evaluated pre-clinically to complete the necessary lead optimization and efficacy testing before initiating IND enabling studies, a prerequisite for seeking FDA IND approval for human clinical trial entry.
For further information: Jakub Staszak-Jirkovsky, Chief Executive Officer, 720.859.4048, jakub@machavert.com